medmix Drug Delivery (Haselmeier) launches its passive needle safety device SicuroJect™
medmix Drug Delivery (Haselmeier) launches its passive needle safety device SicuroJect™ at Pharmapack Europe in Paris, further expanding its product portfolio.
medmix Drug Delivery introduces SicuroJect, a disposable passive needle safety device for subcutaneous injection. The device is intended for use by patients, healthcare professionals or caregivers to prevent needle stick injuries after use. SicuroJect is being developed in two variants for a standard 1 mL long or 2.25 mL pre-filled glass or plastic syringe. Haselmeier offers SicuroJect as an off-the-shelf solution and as a full-service platform including combination product development, design verification, final assembly, secondary packaging, labeling and serialization, depending on the customer’s needs.
SicuroJect is intuitive and easy to use because it uses the same injection technique as a pre-filled syringe without the need for an additional step to activate the safety feature. To prevent needle stick injuries after use, the safety feature is automatically activated at the end of injection. The mechanism also prevents accidental activation. The ergonomic design allows an easy one-handed activation, and optional add-on finger flanges improve control for users of all ages and for high-viscosity drugs. The transparent barrel cover allows the patient to easily see if the medication has been completely administered.
“With SicuroJect, we are further expanding our product portfolio, adding a fast and economical solution for pre-filled syringes”, said Frank Leipold, Vice President Product Management at Haselmeier. “Our collaboration with AARDEX Group and other partners enables a comprehensive offering for our customers from clinical trials to market launch.” Chris Muenzer, Vice President of Innovation and Development at Haselmeier, adds: “As a second step to improve usability and patient experience, it is easy to upgrade from SicuroJect to PiccoJect™, our fully featured 2-step autoinjector.”
Like PiccoJect and D-Flex™, SicuroJect can also be provided with an RFID label. This makes it possible to collect data on device use, which can be tracked and evaluated thanks to our collaboration with AARDEX Group. Their MEMS® Adherence Software helps to understand the collected data and improve patient adherence.
Some facts and figures about SicuroJect:
- Off-the-shelf device ready for fast market entry of the drug without modification
- Designed for low-to-high-speed assembly lines without interfering with the syringe fill and finish process
- Compatibility to any standard 1mL long or 2.25 mL glass or plastic pre-filled syringe with small round or cut flange
- Adaptable to customers’ branding: Different color options for plunger, color badge and optional add-on finger flanges
- Committed to sustainability: Lower carbon footprint as a standard offering: Utilizing materials with sustainable feedstocks
- A full-service platform including combination product development, design verification, final assembly, secondary packaging, labeling and serialization
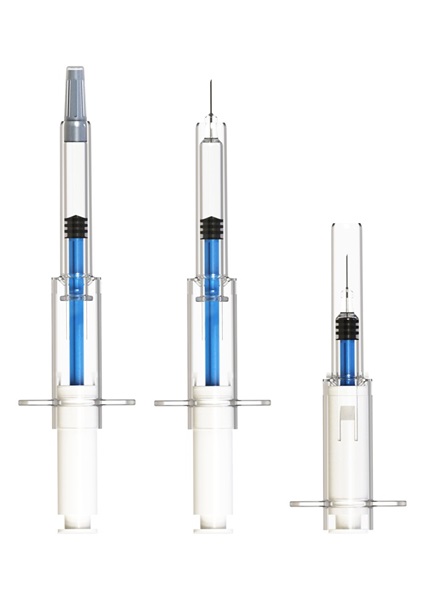 Figure 1: SicuroJect before use with needle cap (left), before use without needle cap (middle) and after use (right), after the safety feature has been automatically activated and the needle has been retracted
Figure 1: SicuroJect before use with needle cap (left), before use without needle cap (middle) and after use (right), after the safety feature has been automatically activated and the needle has been retracted
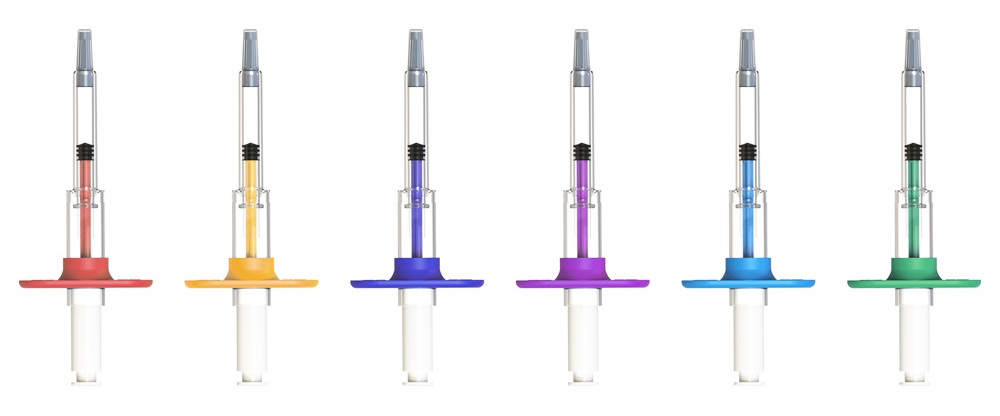 Figure 2: SicuroJect can be easily adapted to customers’ needs
Figure 2: SicuroJect can be easily adapted to customers’ needs
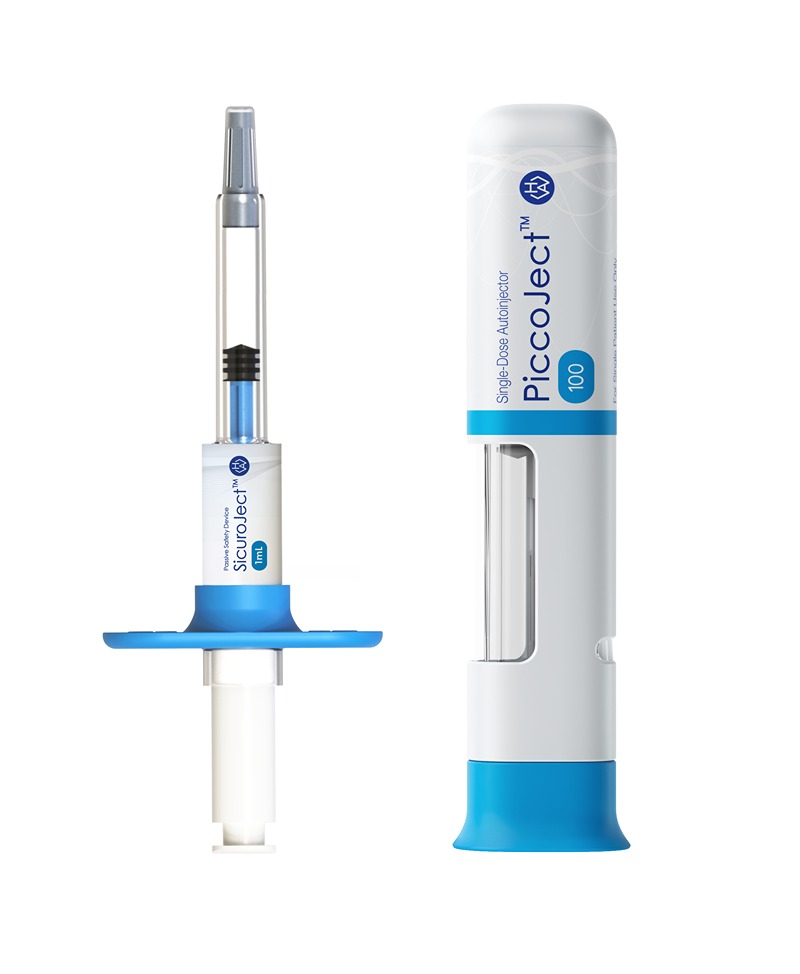 Figure 3: Easy upgrade from SicuroJect to the fully featured 2-step autoinjector PiccoJect to further enhance usability and patient experience
Figure 3: Easy upgrade from SicuroJect to the fully featured 2-step autoinjector PiccoJect to further enhance usability and patient experience
About medmix
medmix is a global leader in high-precision delivery devices. We occupy leading positions in the healthcare, consumer and industrial end-markets. Our customers benefit from a dedication to innovation and technological advancement that has resulted in over 900 active patents. Our 14 production sites worldwide together with our highly motivated and experienced team of nearly 2’600 employees provide our customers with uncompromising quality, proximity and agility. medmix is headquartered in Baar, Switzerland. Our shares are traded on the SIX Swiss Exchange (SIX: MEDX). www.medmix.swiss
Inquiries:
Media Relations, medmix communications@medmix.com
Investor Relations, medmix investorrelations@medmix.com
This document may contain forward-looking statements including, but not limited to, projections of financial developments, market activity, or future performance of products and solutions containing risks and uncertainties. These forward-looking statements are subject to change based on known or unknown risks and various other factors that could cause actual results or performance to differ materially from the statements made herein.