medmix Drug Delivery (Haselmeier™) and AARDEX® Group join Forces to Improve Self-Administration of Injectable Drugs in Clinical Trials
Stuttgart – medmix Drug Delivery (Haselmeier) and AARDEX Group announced today a collaboration, combining Haselmeier´s D-Flex™ Logbook with AARDEX Group’s Medication Adherence software and Hardware ecosystem.
Clinical testing during new drug development is costly and time intensive for pharmaceutical and biotech companies. The need for solutions, helping them to shorten testing time and increase data quality are two of the main factors determining overall cost and success of newly developed drugs. As a result, the future of clinical trials is becoming more reliant on connected medical devices, collecting data at the point of care, reducing the need for costly patient visits and cumbersome manual data acquisition. Therefore, Haselmeier, a medmix Brand, and AARDEX Group have joined forces to tackle the challenge of managing patient adherence during clinical trials.
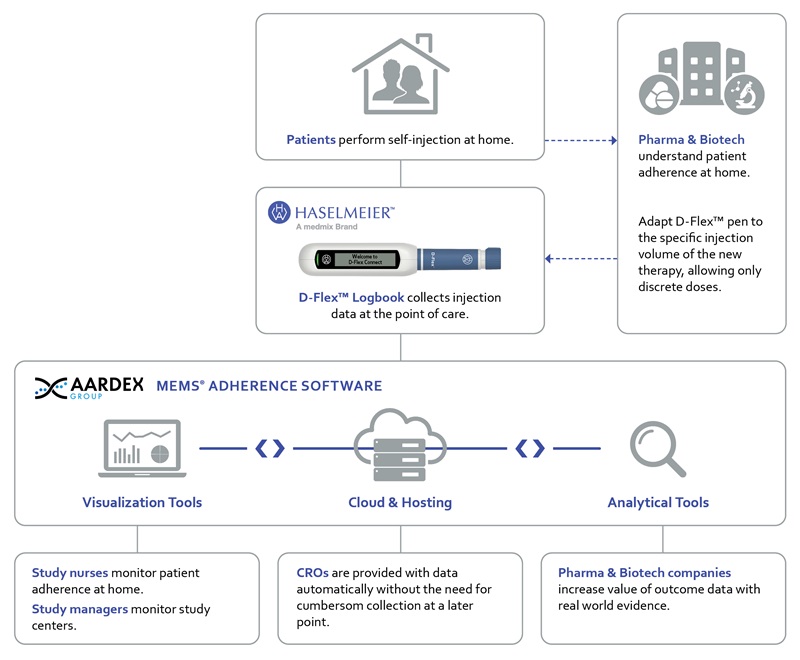 Figure 1: Overview of the combined systems D-Flex Logbook and MEMS Adherence Software in a clinical setting
Figure 1: Overview of the combined systems D-Flex Logbook and MEMS Adherence Software in a clinical setting
Haselmeier developed the D-Flex Logbook, a wireless connected drug delivery solution. Now this solution can be easily integrated into AARDEX Group’s adherence software, MEMS AS®. This electronic data capture system has been successfully utilized to collect patient adherence data from digitally enabled devices such as pillboxes and drug containers for over 20 years. The combination of the two systems allows researchers to understand and manage patient adherence in clinical trials testing self-injection therapies.
Given AARDEX Group’s and Haselmeier´s decades long experience in the pharma industry, the collaboration comes naturally, complementing one another’s offering in clinical testing. This evidence-based approach can make the difference between failed and successful clinical trials.
About MEMS® technology
AARDEX medication adherence solutions are based on the MEMS Adherence Software, MEMS AS, integrating visualization and analytical tools which process dosing history data recorded by smart pharmaceutical packages or smart devices. Furthermore, it allows to apply advanced analytics, helping to identify patients who need additional support at home.
About D-Flex Logbook
Based on a proven technology, the D-Flex Logbook was successfully used in a clinical trial with 75 patients. The learnings from that trial translated into the new and improved D-Flex Logbook1. It consists of the disposable D-Flex injection pen and the connected cap, which replaces the standard cap of the pen. This set-up does not impact patient behavior and results in no additional training. The connected cap tracks injection dose, temperature, and time of up to 1000 injection events. It can securely transfer data in real-time to MEMS Connect or any other pre-existing data management system via Bluetooth Low Energy.
The D-Flex injection pen is at the heart of this connected solution. It allows for simplified adaption of specific injection dose volumes by changing only one component. This saves time and cost during clinical testing, especially with dose ranging studies, because it allows to use the same pen for both, clinical testing and product launch, eliminating the need for additional equivalence and human factor studies, reducing time to market.
The connected cap is currently the only available device that identifies the actual dose delivered by the pen by comparing the position of the plunger before and after each injection. Six LEDs emit an IR signal which is altered by the plunger position. 16 IR sensors then detect the incoming signal. The delta between the two states identifies the relative movement of the plunger, delivering the actual expelled dose during an injection. This way the cap can also calculate and display the remaining volume in the cartridge.
1 Diabetes Care. 2019 Jun;42(6):1129-1131. doi: 10.2337/dc18-1631. Epub 2019 Mar 12.: Nonadherence to Insulin Therapy Detected by Bluetooth-Enabled Pen Cap Is Associated With Poor Glycemic Control
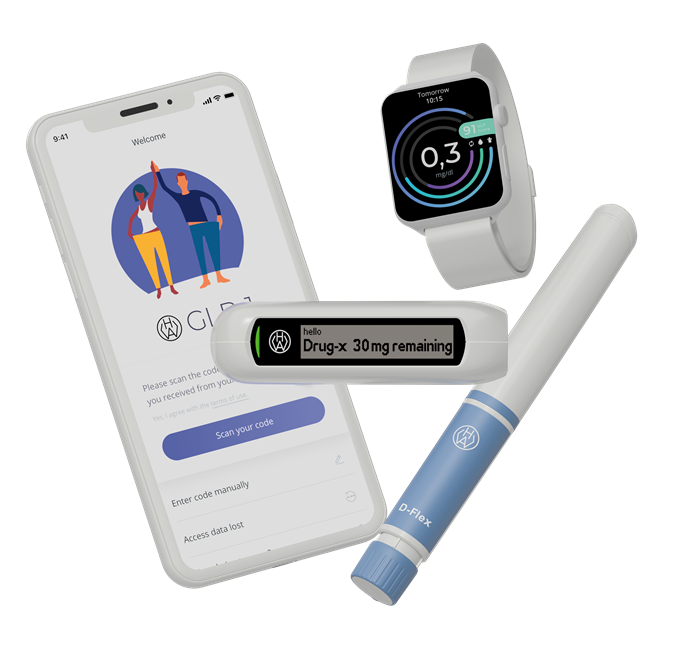 Figure 2: The D-Flex Logbook has been integrated into an ecosystem and wearable via bluetooth.
Figure 2: The D-Flex Logbook has been integrated into an ecosystem and wearable via bluetooth.
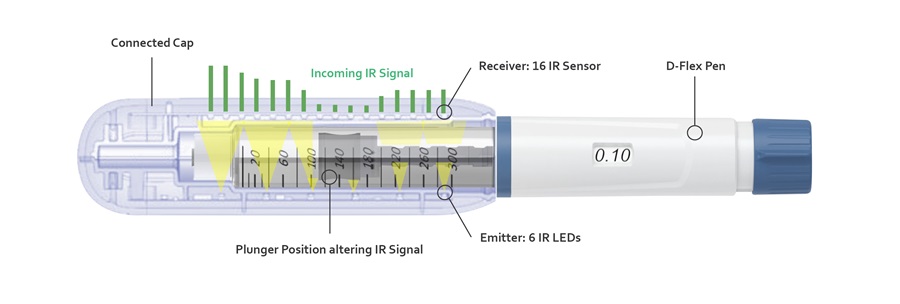 Figure 3: Cross-section of the Connected Cap, which is attached to a D-Flex pen.
Figure 3: Cross-section of the Connected Cap, which is attached to a D-Flex pen.
About AARDEX®
AARDEX Group is the global leader in digital solutions to measure and manage medication adherence. With operations in Belgium, Switzerland, and the U.S., AARDEX develops and markets digital solutions for adherence-enhancing strategies in clinical trials, research settings, and professional healthcare systems. AARDEX is the central actor of a complete ecosystem that combines its MEMS Adherence Software with a wide range of smart packages and devices that measure patient adherence across all routes of drug administration. AARDEX’s vision is to continuously innovate in data-driven medication adherence solutions to enhance digital therapeutics and patient empowerment. www.aardexgroup.com
About Haselmeier™, a medmix Brand
Haselmeier, the drug delivery device business of medmix, designs, develops and manufactures advanced drug delivery systems such as pen injection systems and autoinjectors. Patient comfort and customers’ needs are always at the heart of the company’s practices.
With its broad portfolio of technologies and services, Haselmeier delivers user-friendly injection systems that enable patients to self-administer their medication reliably and accurately.
Haselmeier is known for its excellent and long-standing track record in providing these innovative drug delivery devices based on its proprietary IP business model. The company collaborates closely with its customers in the pharmaceutical and biopharmaceutical industries.
About medmix
medmix is a global leader in high-precision delivery devices. We occupy leading positions in the healthcare, consumer and industrial end-markets. Our customers benefit from a dedication to innovation and technological advancement that has resulted in over 900 active patents. Our 13 production sites worldwide together with our highly motivated and experienced team of nearly 2’000 employees provide our customers with uncompromising quality, proximity and agility. medmix is headquartered in Baar, Switzerland. Our shares are traded on the SIX Swiss Exchange (SIX: MEDX). www.medmix.swiss
Inquiries:
Media Relations: Peter Trampert, Senior Communications Manager
Investor Relations: Sheel Gill, Head of Investor Relations
This document may contain forward-looking statements including, but not limited to, projections of financial developments, market activity, or future performance of products and solutions containing risks and uncertainties. These forward-looking statements are subject to change based on known or unknown risks and various other factors that could cause actual results or performance to differ materially from the statements made herein.